Antibody-drug conjugates (ADCs) have emerged as a promising class of cancer treatments, gaining increasing attention in recent years. These drugs typically consist of three key components: a monoclonal antibody, a cytotoxin, and a linker. The antibody specifically targets tumor cells, the cytotoxin exerts cell-killing effects, and the linker connects and releases the toxin.
Since trastuzumab emtansine (T‑DM1) entered the field of solid tumor treatment in 2013, 17 ADC drugs have been approved globally. However, a significant challenge in treating solid tumors with ADCs is the heterogeneous expression of target antigens within tumor tissues or metastases, where cells may concurrently display high, low, or no antigen expression, which affects ADC efficacy. Fortunately, the ADC bystander effect offers a promising solution to this challenge, potentially expanding the therapeutic reach of ADCs in solid tumors, especially metastases. In the following sections, we will explore the concept of the bystander effect and its critical role in ADC therapy.
The Concept of the ADC Bystander Effect
The bystander effect refers to the ability of ADCs to kill not only targeted tumor cells (Ag+) but also neighboring, antigen-negative (Ag-) cells, regardless of their antigen expression status. This effect depends on the biochemical properties of the ADC's linker and cytotoxin, allowing the payload to diffuse and kill nearby cells.
The Mechanism of the ADC Bystander Effect
The bystander effect of ADCs involves several key steps:
- 1. Antibody–Antigen Binding: The ADC binds specifically to tumor cells via its antibody component, which recognizes and attaches to a target antigen on the cell surface. In ADCs with bystander effect, the cytotoxic payload may even be released before the ADC is internalized, increasing the likelihood of diffusion into nearby cells.
- 2. Internalization: Once bound, the ADC is internalized by the tumor cell through endocytosis. Inside the lysosome, the ADC is broken down, leading to the release of the cytotoxic payload.
- 3. Payload Diffusion: Non-polar payloads are membrane-permeable and can diffuse out of the target cell into neighboring tumor cells, regardless of their antigen expression. This diffusion underpins the bystander effect and contributes to enhanced antitumor efficacy, particularly in tumors with heterogeneous antigen expression.
Many factors influence the bystander effect.
First, the chemical stability of the bond between the antibody and the linker is a crucial determinant. In ADCs lacking a bystander effect, this bond is highly stable, ensuring that the cytotoxic payload is not released prematurely. In contrast, ADCs designed for bystander effect may have less stable bonds, allowing payload release before or during internalization, thereby enabling diffusion into neighboring cells.
Second, the type and stability of the linker itself also play a pivotal role. Linkers are generally classified as cleavable or non-cleavable. Cleavable linkers—such as pH-sensitive hydrazones or enzyme-cleavable dipeptides—can be broken down either extracellularly or intracellularly to release the payload. Non-cleavable linkers, typically covalent bonds like thioethers, require internalization and lysosomal degradation before releasing the cytotoxin.
Third, the physicochemical properties of the payload significantly influence the extent of the bystander effect. Non-polar, membrane-permeable payloads can readily diffuse out of target cells and affect nearby tumor cells. In contrast, highly polar payloads struggle to cross the cell membrane. A notable example is trastuzumab emtansine (T-DM1): although its payload is not highly polar, incomplete cleavage leaves a positively charged lysine attached, preventing membrane permeability and eliminating the bystander effect.
Finally, when a target tumor cell is destroyed by the ADC, the intracellular payload may be passively released into the surrounding tumor microenvironment. This post-lysis diffusion can contribute to bystander killing of adjacent tumor cells, further enhancing the overall therapeutic impact of the ADC.
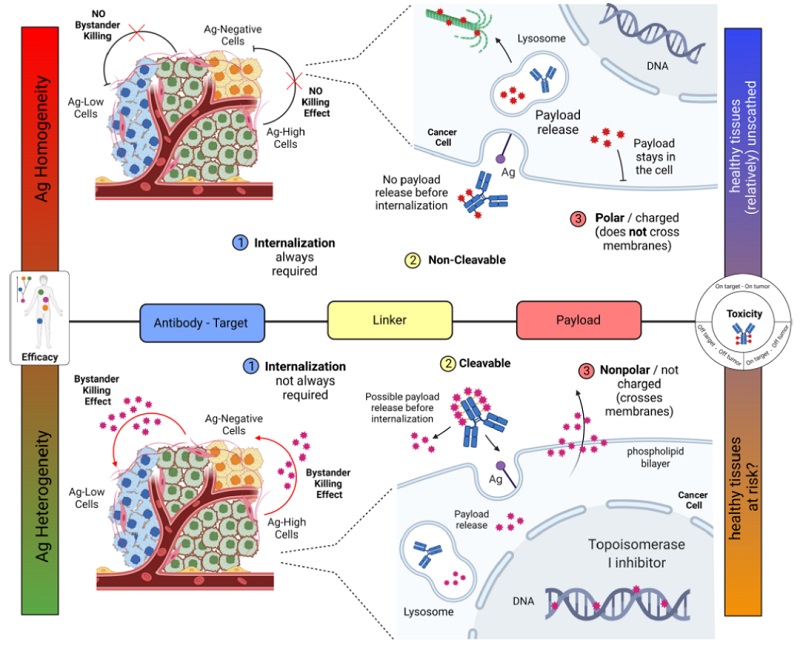
Figure 1. The mechanism of action of ADC without bystander effect (top) and with bystander effect (bottom) (doi: 10.1007/s11912-022-01266-4)
FDA approved ADCs and Bystander Effect
Of the ADCs approved by the FDA, four use payloads capable of bystander effects: Enhertu (HER2/DXd), Trodelvy (TROP-2/SN-38), Padcev (Nectin-4/MMAE), and Tivdak (Tissue factor/MMAE).
Enhertu (HER2/DXd)
Enhertu (trastuzumab deruxtecan, T-DXd) consists of trastuzumab (targeting HER2), toxin DXd (topoisomerase I inhibitor), and a GGFG tetrapeptide linker, which is linked to the cysteine of the antibody amino acid residues.
The GGFG linker is specifically cleaved by highly expressed lysosomal proteases in tumors, releasing the cytotoxic DXd payload. The released DXd toxin has strong membrane permeability, allowing it to diffuse from the targeted cancer cells into nearby tumor cells. This mechanism enables DXd to kill adjacent cancer cells, thereby inducing a "bystander effect" and extending the therapeutic impact beyond the directly targeted cells.
Experiments were conducted using a mixture of HER2-positive (HER2+) human gastric cancer cells, NCI-N87, and HER2-negative (HER2-) human breast cancer cells, MDA-MB-468. The results showed that T-DXd was effective not only in killing HER2+ NCI-N87 cells but also in eliminating neighboring HER2- MDA-MB-468 cells.
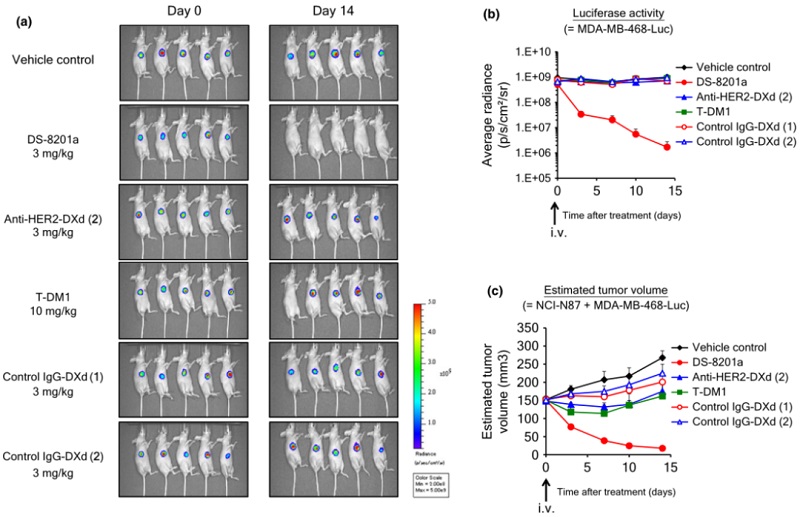
Figure 2 Bystander effect of T-DXd in (HER2+)NCI-N87 and (HER2-)MDA-MB-468 xenografts (doi: 10.1111/cas.12966)
In xenograft experiments conducted in mice, T-DXd significantly reduced the signal from luciferase-expressing MDA-MB-468 cells, indicating that T-DXd inhibited HER2- cell populations. Notably, T-DXd was ineffective against HER2- cells located at a distance from the HER2+ cell inoculation site, suggesting that the bystander effect is localized. This implies that non-specific systemic toxicity and toxicity in normal tissues far from HER2+ cells may be relatively low.
Additionally, a recent in vivo study evaluated a novel imaging system using fluorescent nanoparticles as immunostaining markers to visualize the distribution of DXd within tumor tissues. The researchers found that DXd was evenly distributed across both HER2+ and HER2- regions of the tumor.
Trodelvy (TROP-2/SN-38)
Trodelvy (Sacituzumab govitecan) is an ADC that targets TROP-2-expressing cancer cells, delivering the topoisomerase I inhibitor SN-38 via a hydrolyzable linker, resulting in a bystander effect.
In co-culture experiments, sacituzumab govitecan demonstrated a bystander effect, effectively killing TROP-2-negative ovarian cancer cells when co-cultured with TROP-2-positive cells. [4]
Padcev (Nectin-4/MMAE)
Padcev (enfortumab vedotin-ejfv) is an ADC comprised of a Nectin-4-directed IgG1 antibody and a microtubule-disrupting agent (MMAE), which is attached to the antibody via a protease-cleavable linker.
In co-culture experiments with Nectin-4-positive and Nectin-4-negative tumor cells, enfortumab vedotin demonstrated a bystander effect by releasing the membrane-permeable MMAE payload, effectively killing the Nectin-4-negative cells.
Tivdak (Tissue factor/MMAE)
Tisotumab vedotin (Tivdak™) consists of tisotumab, a monoclonal antibody targeting human tissue factor (TF), which is covalently linked via a protease-cleavable peptide linker to MMAE.
In co-culture experiments with TF-positive and TF-negative tumor cells, Tisotumab vedotin demonstrated a bystander effect, exhibiting cytotoxicity in both cell types.
Conclusion
Preclinical studies have shown that in addition to T-DXd, many other ADCs also have bystander effects, such as sacituzumab govitecan (SG), tisotumab vedotin (TV), enfortumab vedotin (EV), anetumab ravtansine (BAY 94–9343).
In addition, a large number of clinical trials have also begun to investigate bystander effects, which have expanded the clinical indications of ADCs. There are currently five ADC drugs for the treatment of solid tumors, including T-DM1, T-DXd, SG, TV and EV. Although the bystander effect seems to be very meaningful for efficacy, whether it will bring toxicity also needs to be considered. More and more preclinical/clinical trials are beginning to focus on these issues to guide the design of next-generation ADCs.
Biopharma PEG, a professional PEG derivatives supplier, is dedicated to being your most reliable partner to manufacture and supply high purity ADC linkers (PEG liners) & Click Chemistry Reagents. We offer the full range of PEG derivative development services and provide the most comprehensive media for conjugation research.
References:
[1] Giugliano F, Corti C, Tarantino P, Michelini F, Curigliano G. Bystander effect of antibody-drug conjugates: fact or fiction? Curr Oncol Rep. 2022 Mar 19. doi: 10.1007/s11912-022-01266-4.
https://pubmed.ncbi.nlm.nih.gov/35305211/
[2] Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016 Jul;107(7):1039-46. doi: 10.1111/cas.12966.
https://pubmed.ncbi.nlm.nih.gov/27166974/
[3] Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer. 2017 Dec 5;117(12):1736-1742. doi: 10.1038/bjc.2017.367.
https://www.nature.com/articles/bjc2017367
[4] Perrone E, Lopez S, Zeybek B, Bellone S, Bonazzoli E, Pelligra S, Zammataro L, Manzano A, Manara P, Bianchi A, Buza N, Tymon-Rosario J, Altwerger G, Han C, Menderes G, Ratner E, Silasi DA, Azodi M, Hui P, Schwartz PE, Scambia G, Santin AD. Preclinical Activity of Sacituzumab Govitecan, an Antibody-Drug Conjugate Targeting Trophoblast Cell-Surface Antigen 2 (Trop-2) Linked to the Active Metabolite of Irinotecan (SN-38), in Ovarian Cancer. Front Oncol. 2020 Feb 12;10:118. doi: 10.3389/fonc.2020.00118. PMID: 32117765; PMCID: PMC7028697.
Related articles:
[1] ADC Drugs Global Sales of 2021 and Future Prospects
[2] Learn More About ADCs From Its Structure
[3] How To Choose The Best ADC Linker?
[4] FDA Approved Antibody-Drug Conjugates (ADCs) Up To 2022

