PEGs are used in many applications as they are generally considered as safe (GRAS), are biologically inert and highly water soluble. The covalent/noncovalent attachment of PEG chains to therapeutic agents such as small molecule drugs, peptides, and nucleic acids is a well-established procedure to optimize the pharmacokinetics and pharmacodynamics of drugs and the above strategy is also known as PEGylation.
PEGylation improves drug stability, reduces nonspecific protein absorption and macrophage uptake, and significantly prolongs circulation time due to the stealth effect of PEG. Moreover, the increased molecular weight and hydrodynamic sizes reduce renal filtration, enabling a long-term action of drugs and thus a significant reduction in dosing frequency. To this purpose, PEG has been chemically modified by introducing a variety of functional groups to synthesize tailored PEG derivatives, making this polymer even more suitable for clinical drug development.
There are two main types of PEGylation: PEGylation with conventional polydisperse PEG, a polymer mixture with an average molecular weight and a polydispersity index (PDI) above 1.1, and PEGylation with monodisperse(discrete) PEG, which has a precise molecular weight with a PDI of 1.0.
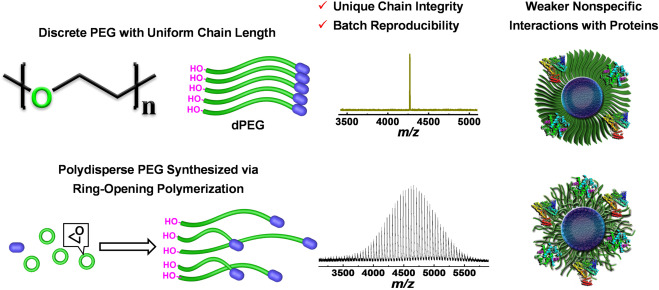
Figure 1. Comparison of discrete PEG with conventional polydisperse PEG [2]
To date, over 40 PEGylated drugs have been approved by the FDA, most of which utilize polydisperse PEGs.
Polydisperse PEGylation
Polydisperse PEGs have been prepared by acid, base, or metal-catalyzed ring-opening polymerization of ethylene oxide. They are easy to prepare and inexpensive.
In 1970, Abuchowski et al. successfully attached PEG to bovine serum albumin (BSA) and discovered that it significantly reduced its immunogenicity. This breakthrough made PEG functionalization one of the most widely used techniques for modifying pharmaceutical molecules and improving drug delivery.
In the early 1990s, researchers found that PEGylated liposomes, when administered intravenously, significantly prolonged drug blood circulation. PEG forms a hydration layer on the surface of the liposome, enhancing the stability of the phospholipid bilayer and reducing the adsorption of plasma proteins. At the same time, PEG can significantly reduce the recognition of liposomes by mononuclear phagocytic system (MPS), and to a certain extent, shield the immune clearance of the host.
Since the pioneering work of Davis and Abuchowski, several PEGylated products have come to the market. In 1991, the FDA approved ADAGEN, the first PEGylated protein therapy, for treating adenosine deaminase deficiency. Other PEGylated products such as Doxil® for cancer treatment, Pegasyss® and PEG-Intron® for Hepatitis C, SonoVue® for ultrasound imaging, and Comirnaty®, Pfizer's COVID-19 vaccine have been approved by FDA successively.
Although polydisperse PEG has long been considered to have the advantages of non-toxicity, non-immunogenicity and non-antigenicity, increasing evidence is challenging this view. PEGylation with polydispersed PEG is also associated with several drawbacks.
The polydisperse nature of conventional PEG not only complicates the synthesis and purification processes of PEGylated drugs but also leads to heterogeneity in the final products, which affects the consistency and reproducibility of drug quality. Additionally, PEG's nondegradable properties can enhance immunogenic and antigenic responses, reducing therapeutic efficacy and causing unwanted side effects.
A study by Lai from the University of North Carolina showed a 360-fold increase in the number of people with anti-PEG antibodies between 1984 and 2016. This rise is largely attributed to the expanding use of PEG, not only in cosmetics and pharmaceuticals but also as a key component in biologic medicines.
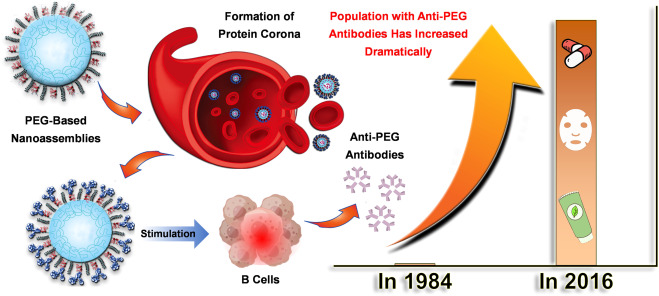
Figure 2. PEG-containing products generate anti-PEG antibodies. [2]
The presence of anti-PEG antibodies poses two significant risks:
- Immunogenicity: PEG antibodies increase the likelihood that PEGylated drugs will be recognized and captured by the mononuclear phagocyte system (MPS), accelerating their clearance from the bloodstream — known as the "ABC effect" — and thereby reducing the drugs' efficacy. For instance, in a clinical trial of PEGylated uricase for patients with chronic refractory gout, 5 out of 13 patients experienced rapid drug clearance after injection due to the presence of low levels of anti-PEG antibodies (IgG and IgM).
- Antigenicity: PEG antibodies can activate the complement system, triggering hypersensitivity reactions (HSRs), which may result in severe allergic reactions, including anaphylactic shock or, in extreme cases, death. For example, allergic reactions to COVID-19 RNA vaccines developed by Pfizer (BNT162b2) and Moderna (mRNA-1273) have been linked to PEG-containing lipid formulations.
Monodisperse (Discrete) PEGylation
To address the limitations of polydisperse PEG products, techniques for producing monodisperse PEG have been developed. Unlike conventional polydisperse PEG, monodisperse PEG has uniform chemical structures with precise molecular weights. It demonstrates improved solubility, biocompatibility, and reduced immunogenicity. Additionally, the ability to introduce functional groups or modifications to the PEG chain has allowed for greater versatility in their use as drug delivery vehicles, imaging agents, and more.
The key advantage of using monodisperse PEG for biopharmaceuticals lies in the ability to fully control molecular weight, chain length, and configuration. This allows for the optimization of a drug's pharmacokinetic properties and the fine-tuning of its chemical characteristics to target specific sites of action more effectively. As new oligonucleotides, peptides, proteins, and antibody fragments continue to emerge, monodisperse PEG offers significant potential for enhancing their diagnostic, imaging, and therapeutic applications.
An example of this technology in action is Movantik (naloxegol), a monodisperse PEGylated small-molecule drug developed by AstraZeneca. Approved by the FDA in 2014 for treating opioid-induced constipation, Movantik's monodisperse PEGylation (m-PEG7 linker) improves its solubility, bioavailability, and pharmacokinetics, making it more effective than conventional alternatives.
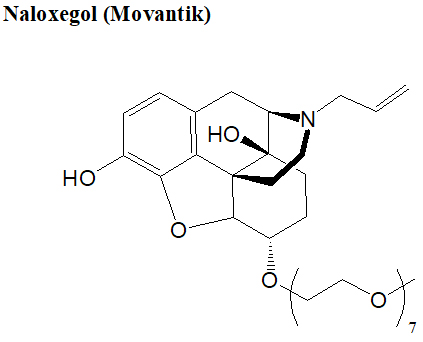
Figure 3. Structure of Movantik
Biopharma PEG's monodisperse PEG products are synthesized from high-purity building blocks through stepwise reactions to achieve specific molecular weights and functional groups suited for a wide variety of applications. The PEG products feature methoxy, alcohol, and carboxylic acid end-capping, with protective groups like Fmoc and Boc, and functional groups for conjugating to various molecules such as amines, thiols, and carboxylic acids, etc.
References:
1. Yang Q, Jacobs TM, McCallen JD, Moore DT, Huckaby JT, Edelstein JN, Lai SK. Analysis of Pre-existing IgG and IgM Antibodies against Polyethylene Glycol (PEG) in the General Population. Anal Chem. 2016 Dec 6;88(23):11804-11812. doi: 10.1021/acs.analchem.6b03437. Epub 2016 Nov 16. PMID: 27804292; PMCID: PMC6512330.
2. Jie Cen, Mingxuan Hou, Shiyong Liu, Discrete polyethylene glycol derivatives as a potent impetus for next-generation biomedicines, Giant, Volume 15, 2023, 100169, ISSN 2666-5425, https://doi.org/10.1016/j.giant.2023.100169.
3. Striving for Uniformity: A Review on Advances and Challenges To Achieve Uniform Polyethylene Glycol, Cláudia Bento, Marianna Katz, Maria M. M. Santos, and Carlos A. M. Afonso, Organic Process Research & Development 2024 28 (4), 860-890, DOI: 10.1021/acs.oprd.3c00428
Related Articles:
FDA Approved PEGylated Drugs By 2024
PEG Linkers & Their Applications
The Difference Between Monodisperse and Polydisperse Polymers
Monodispersed PEGs for Your ADC & PROTAC Development

