Alzheimer's disease (AD) is the most common neurodegenerative disorder leading to progressive cognitive decline with pathological hallmarks of senile plaque and neurofibrillary tangle formation in the brain.
Recently marketed new drugs, including monoclonal antibody therapies such as Lecanemab (Leqembi) and Donanemab (Kisunla) that target β-amyloid (Aβ), are not cures for Alzheimer's disease, while these drugs have been shown to modestly slow worsening symptoms of Alzheimer's disease. With the deepening of genetic research on AD, gene therapy has emerged as one of the most promising curative approaches, with small interfering RNA (siRNA) gene therapy being a current research focus.
siRNA functions by targeting the mRNA of pathogenic genes, inducing its degradation or inhibiting translation, thereby reducing the production of abnormal proteins. In AD, siRNA primarily targets the following pathological pathways:
- ◆ Targeting Amyloid Precursor Protein (APP): siRNA can silence the expression of the APP gene, reducing the production of its cleavage product, β-amyloid (Aβ), thereby inhibiting the formation of amyloid plaques.
- ◆ Modulating Tau Protein Pathology: Research indicates that tau protein phosphorylation exhibits a dependency-regulated pattern, where the phosphorylation of key sites such as T50, T69, and T181 can regulate the phosphorylation of other sites, thereby controlling the overall phosphorylation level of tau protein, which is crucial for the pathogenesis of AD.
siRNAs do not permanently modify the genome, exhibiting strong targeting capabilities, and presenting fewer side effects. However, its clinical application is limited by poor in vivo stability, off-target effects, and the challenge of delivery across the blood-brain barrier (BBB). Nanoparticles are one method for delivering siRNA. Various nanoparticles, such as polyethylene glycol-modified nanoparticles, magnetic nanoparticles, solid lipid nanoparticles, peptide-conjugated nanoparticles, viral glycoprotein-conjugated nanoparticles, and micellar nanoparticles, are being developed to enhance siRNA's ability to cross the BBB and improve therapeutic specificity, thereby advancing its potential as a viable treatment option for AD.
Clinical Status of siRNA-Based AD Drugs
Mivelsiran (ALN-APP)
Mivelsiran is an investigational, intrathecally administered RNAi therapeutic targeting amyloid precursor protein (APP) in development for the treatment of AD and cerebral amyloid angiopathy (CAA). It is designed to decrease APP mRNA in the central nervous system (CNS) and thereby may reduce synthesis of APP protein and all downstream intracellular and extracellular APP-derived cleavage products, including amyloid beta (Aβ).
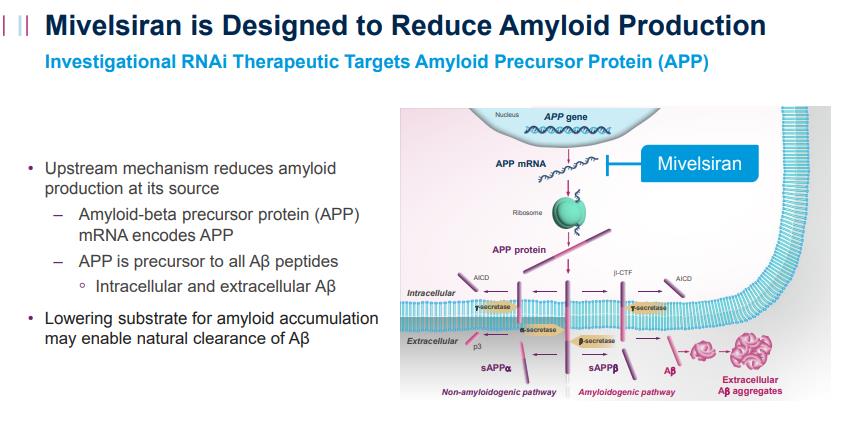
Mechanism of Action of Mivelsiran (Source: Alnylam Pharmaceuticals Website [1])
In Phase 1 clinical trials, Mivelsiran, administered every six months, consistently reduced APP protein levels in the cerebrospinal fluid (CSF) of participants, achieving an average reduction of 73.3% after 12 months. Alnylam has stated that Mivelsiran shows the potential to become a “best-in-class” therapy, with plans to initiate Phase 2 clinical trials in 2025.
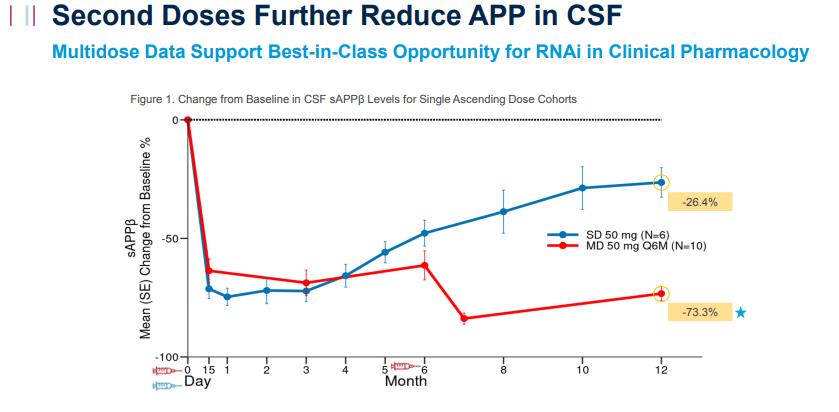
Mivelsiran Significantly Reduces APP Protein Levels in Participants' Cerebrospinal Fluid (Source: Alnylam Pharmaceuticals Website [1])
BIIB080
BIIB080 is designed to target microtubule-associated protein tau (MAPT) mRNA and reduce production of tau protein. Unlike small molecules or antibody drugs, BIIB080 not only targets MAPT mRNA to prevent tau protein production, but also lowers the levels of soluble tau protein in the cerebrospinal fluid of early Alzheimer's disease patients. More importantly, it is the first therapy to reduce the accumulation of pathological tau protein.
It is worth noting that although BIIB080, as the first ASO therapy in the AD field, has not yet shown a significant difference in preliminary efficacy data compared to antibody drugs such as Lecanemab and Donanemab, its unique drug characteristics suggest a more promising clinical application outlook.
BIIB080 achieves high specificity regulation by precisely targeting mRNA, effectively reducing off-target risks. Its long half-life supports extended dosing intervals, potentially extending treatment frequency from the current monthly injections of antibody drugs to once every six months or even annually. This offers important clinical value for managing neurodegenerative diseases that require long-term treatment.
The Phase 2 clinical trial of BIIB080 is ongoing, and its effects on disease progression and cognitive function improvement in mild AD patients are yet to be studied.
References:
[1] https://alnylampharmaceuticalsinc.gcs-web.com/static-files/cb95ec89-97ad-423f-aa9e-393e73ae4159
[2] https://investors.biogen.com/news-releases/news-release-details/new-data-biogens-investigational-antisense-oligonucleotide-aso

