On January 17, 2025, the U.S. FDA announced the approval of Datroway (datopotamab deruxtecan or Dato-DXd), an antibody-drug conjugate (ADC) jointly developed by AstraZeneca and Daiichi Sankyo. The drug is approved for the treatment of adult patients with unresectable or metastatic hormone receptor (HR)-positive, HER2-negative breast cancer who have received prior endocrine-based therapy and chemotherapy. Results from a Phase 3 trial demonstrated that Datroway reduces the risk of disease progression or death by 37% compared to chemotherapy. According to AstraZeneca’s press release, this marks the first FDA approval for Datroway in the United States. Notably, this ADC was ranked byEvaluate as one of the top 10 potential blockbuster R&D projects of 2024.
Dato-DXd is a TROP-2 directed ADC consisting of a humanized anti-TROP-2 IgG1 monoclonal antibody (Mab) linked to a topoisomerase I inhibitor payload by tetrapeptide-based cleavable linker. DXd features a unique mechanism of action, demonstrating 10 times greater potency compared to the commonly used chemotherapy agent irinotecan. Additionally, the high membrane permeability of the Dato-DXd payload after cleavage may allow for a bystander effect and elimination of nearby tumor cells regardless of TROP2 expression.
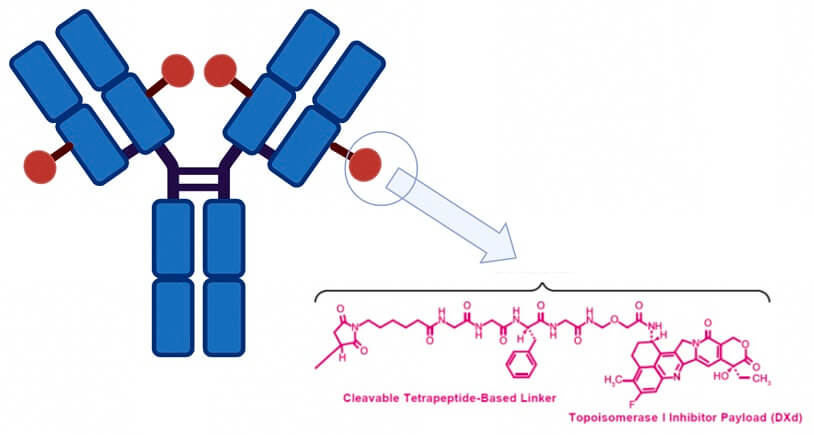
Figure. 1. The structure of Datopotamab Deruxtecan. [2]
The FDA approval was based on the results of the TROPION-Breast01 trial. This global, multicenter, randomized, open-label Phase 3 trial aimed to evaluate the efficacy and safety of Datroway (6 mg/kg administered intravenously every 21 days) compared to investigator’s choice of single-agent chemotherapy (irinotecan, capecitabine, vinorelbine, or gemcitabine). The trial enrolled adult patients with unresectable or metastatic HR-positive, HER2-negative breast cancer who were deemed unresponsive to endocrine therapy and unsuitable for continued treatment, as assessed by the investigators. All participants had previously received at least one chemotherapy regimen for unresectable or metastatic disease.
The major efficacy outcome measures were progression-free survival (PFS), assessed by blinded independent central review (BICR), based on RECIST v1.1 and overall survival (OS). Additional efficacy outcomes included confirmed objective response rate (ORR) and duration of response (DOR) by BICR.
The results showed that the median PFS in the Datroway arm was 6.9 months (95% CI: 5.7, 7.4), compared to 4.9 months (95% CI: 4.2, 5.5) in the chemotherapy arm, with a hazard ratio (HR) of 0.63 (95% CI: 0.52, 0.76) and a two-sided p-value of <0.0001. The median OS was 18.6 months (95% CI: 17.3, 20.1) in the Datroway arm and 18.3 months (95% CI: 17.3, 20.5) in the chemotherapy arm, with an HR of 1.01 (95% CI: 0.83, 1.22), which did not reach statistical significance. The confirmed ORR was 36% (95% CI: 31, 42) in the Datroway arm compared to 23% (95% CI: 19, 28) in the chemotherapy arm, while the median DOR was 6.7 months (95% CI: 5.6, 9.8) and 5.7 months (95% CI: 4.9, 6.8), respectively.
In December 2024, Datroway received its first global approval in Japan for treating adult patients with unresectable or recurrent HR-positive, HER2-negative breast cancer who had previously undergone chemotherapy. That same month, the U.S. FDA granted Datroway Breakthrough Therapy Designation (BTD) for adult patients with locally advanced or metastatic EGFR-mutated non-small cell lung cancer (NSCLC) whose disease had progressed after treatment with an EGFR tyrosine kinase inhibitor (TKI) and platinum-based chemotherapy. In January 2025, the FDA further granted Priority Review for Datroway in the same advanced EGFR-mutated NSCLC population, with a decision expected by Q3 2025.
Datroway Clinical Development Program
A robust global clinical development program is currently in progress, featuring over 20 trials assessing the efficacy and safety of Datroway across multiple cancer types, including NSCLC, triple-negative breast cancer (TNBC), and HR-positive, HER2-negative breast cancer. The program includes seven Phase III trials in lung cancer and five Phase III trials in breast cancer, evaluating Datroway as a monotherapy and in combination with other anticancer treatments in various settings.
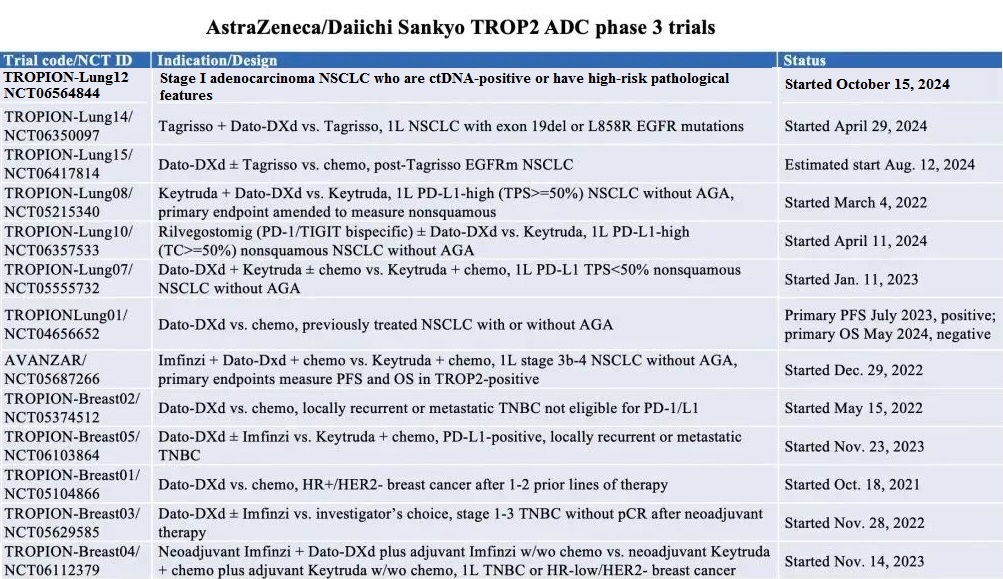
Figure 2. Datroway Phase 3 trials
Emerging TROP-2 Directed ADCs
Frost & Sullivan predicts that the global Trop2 ADC market will reach $25.9 billion by 2030, growing at a remarkable compound annual growth rate (CAGR) of 57.6% from 2022 to 2030. This lucrative market has attracted significant interest, with nearly 60 Trop2 ADC candidates currently in active development worldwide. Among these, three have already received regulatory approval: Trodelvy, Dato-DXd, and SKB264 (approved by China’s NMPA).
Trop2 is a key tumor growth factor highly expressed in several cancers, including breast, gastric, NSCLC, SCLC, colorectal, and pancreatic cancers. It plays a critical role in tumor cell proliferation, invasion, and metastasis, and its overexpression is closely linked to shorter survival and poor prognosis in cancer patients. As a result, targeting Trop2 in cancer treatment is of significant therapeutic interest.
Currently, Trop2 is the second most popular ADC target after HER2, with 16 candidate drugs undergoing clinical development, according to an April study published in Nature Reviews Drug Discovery.
Biopharma PEG is a professional PEG supplier. We can provide high-purity PEG linkers from milligram to kilogram scale in GMP and Non-GMP grade for your ADC development. The use of PEGs as a linker between the antibody and payload molecules allows for higher ADC loading. PEGs create a protective shield that wraps the ADC payload from its microenvironment, improving solubility and stability. Other benefits include reduced aggregation and thus lower immunogenicity, improved pharmacokinetics, increased circulation time and reduced toxicity.
References:
[1] Datroway (datopotamab deruxtecan) approved in the US for patients with previously treated metastatic HR-positive, HER2-negative breast cancer https://www.astrazeneca.com/media-centre/press-releases/2025/dato-dxd-approved-in-us-for-hr-p-breast-cancer.html
[2] Schipilliti FM, Drittone D, Mazzuca F, La Forgia D, Guven DC, Rizzo A. Datopotamab deruxtecan: A novel antibody drug conjugate for triple-negative breast cancer. Heliyon. 2024 Mar 22;10(7):e28385. doi: 10.1016/j.heliyon.2024.e28385. PMID: 38560142; PMCID: PMC10981107.
[3] 3 Big Pharma companies, 33 phase 3 trials: The race for supremacy in an ADC field https://www.fiercepharma.com/pharma/3-big-pharma-companies-33-phase-3-trials-race-supremacy-adc-field#0cca9935-6593-4b4b-896d-a84426d1195c

